|
|
|
|
BIOCHEMISTRY |
Biochemistry is the study of the chemicals within the biological systems of living organisms. The chemicals that make up living things are mostly organic macromolecules belonging to the 4 main groups proteins, nucleic acids, carbohydrates or lipids. These macromolecules are made up from specific monomers as shown in the table below. Between them these four groups make up 93% of the dry mass of living organisms, the remaining 7% comprising small organic molecules (like vitamins) and inorganic ions.
|
Group name |
monomers |
polymers |
|
Proteins |
amino acids |
protein |
|
Nucleic acids |
nucleotides |
n/a |
|
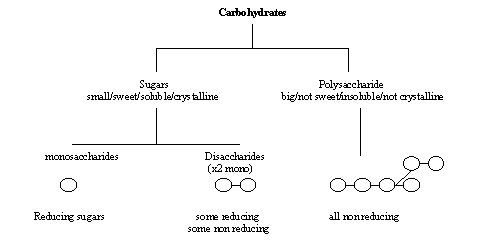
Carbohydrates |
monosaccharides |
polysaccharides |
Lipids are not composed of a repeating monomer, so they do not make polymers and instead are made of fatty acids and glycerol.
|
Group name |
components |
largest unit |
|
lipids |
fatty acids + glycerol |
Triglycerides |
Carbohydrates contain only the elements carbon (C), hydrogen (H) and oxygen (O).
Monosaccharides
All have the formula (CH2O)n, where n is between 3 and 7. Monosaccharides cannot be broken down into sugars of a similar size. Monosaccharides are classified by the number of carbon atoms in the molecule.
There are many monosaccharides, with the same chemical formula (C6H12O6), but different structural formulae. These include fructose and glucose. This is called isomerism.
Monosaccharides can be tested for using Benedict's solutionb as all monosaccharides are reducing sugars they will reduce the Benedict's solutuion and turn it from a clear blue to a brick red percipitate.
Monosaccharides are small and soluble so are perfect for transport around the body. They can pass through the partially permeable membrane of the cells.
Disaccharides
Disaccharides are formed when two monosaccharides are joined together by a glycosidic bond. The reaction involves the formation of the molecule water (H2O):
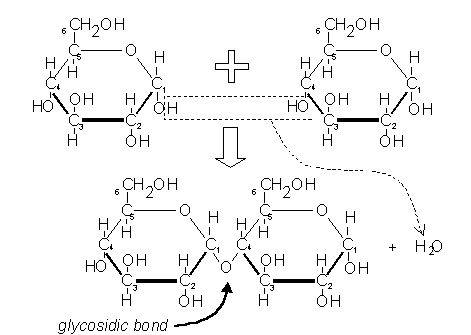 |
The above shows two glucose molecules joining together to form the disaccharide maltose. Because this bond is between carbon 1 of one molecule and carbon 4 of the other molecule it is called a 1-4 glycosidic bond. This kind of reaction, where water is formed, is called a condensation reaction. The reverse process, when bonds are broken by the addition of water (e.g. in digestion), is called a hydrolysis reaction.
There are three common disaccharides:
Detecting disaccharides:
As some disaccharides are reducing sugars and others are non reducing the test is in two parts. First test the disaccharide in Benedicts solution, if the Benedict's solution remains unchanged then the solution could either be a non reducing disaccharide or a polysaccharide. We now need to hydrolise the disaccharide we can do this by boiling it in HCl this will break the disaccharide down into its monosaccharide parts which will now reduce Benedict's in the normal way.
Polysaccharides
Polysaccharides are long chains of many monosaccharides joined together by glycosidic bonds. There are three important polysaccharides: The number of monosaccharides is variable and the chain can be branched or un branched. The chains can be folded making them compact and ideal for storage. The size of the molecules makes them compact and ideal for storage. This isvery helpful as it exerts no osmotic influence on cells and does not diffuse.
Starch is the plant storage polysaccharide. It is insoluble and forms starch granules inside many plant cells. Being insoluble means starch does not change the water potential of cells, so does not cause the cells to take up water by osmosis.
| Glycogen is made by animals as their storage polysaccharide, and is found mainly in muscle and liver. Because it is so highly branched it can be broken down to glucose for energy very quickly. |
Cellulose is only found in plants, where it is the main component of cell walls. It is poly (1-4) glucose, but with a different isomer of glucose. Cellulose contains beta-glucose, in which the hydroxyl group on carbon 1 sticks up. This means that in a chain alternate glucose molecules are inverted.
This tiny difference makes a huge difference in structure and properties. While the a1-4 glucose polymer in starch coils up to form granules, the beta1-4 glucose polymer in cellulose forms straight chains. Hundreds of these chains are linked together by hydrogen bonds to form cellulose microfibrils. These microfibrils are very strong and rigid, and give strength to plant cells.
Lipids are a mixed group of hydrophobic compounds composed of the elements carbon, hydrogen and oxygen. They contain fats and oils (fats are solid at room temperature, whereas oils are liquid)
Lipids are insoluble and their chief function is energy storage. Fat stored in animals can also act as an insulator.
Structure ofLipids
Lipids are the compounds of an alcohol and fatty acids formed by condensation reactions.
The most common alcohol is glycerol. Glycerol has three OH groups which can combine with a fatty acid forming a triglyceride.
| Glycerol is a small, 3-carbon molecule with three hydroxyl groups. |
|
Fatty acids are long molecules with a polar, hydrophilic end and a non-polar, hydrophobic "tail". The formula of a fatty acid can be written as R-COOH. Where the R stands for the remainder of the molecule, the long hydrocarbon chain.
Triglycerides are insoluble in water. They are used for storage, insulation and protection in fatty tissue found under the skin or surrounding organs. They yield more energy per unit mass than other compounds so are good for energy storage. Carbohydrates can be mobilised more quickly, and glycogen is stored in muscles and the liver for immediate energy requirements.
Phospholipids
 |
Phospholipids have a similar structure to triglycerides, but with a phosphate group in place of one fatty acid chain. There may also be other groups attached to the phosphate. Phospholipids have a polar hydrophilic "head" (the negatively-charged phosphate group) and two non-polar hydrophobic "tails" (the fatty acid chains). This mixture of properties is fundamental to biology, for phospholipids are the main components of cell membranes.
|
|
|
|
Waxes
Waxes are formed from fatty acids and long-chain alcohols. They are commonly found wherever waterproofing is needed, such as in leaf cuticles, insect exoskeletons, birds' feathers and mammals' fur.
Steroids
Steroids are small hydrophobic molecules found mainly in animals. They include:
Coming soon ...